【Background】.
Tritium (3H) labeling is an important tool for the study of pharmacokinetic and pharmacodynamic properties of drugs, radioautography, receptor binding and receptor occupancy studies. Tritium gas is the preferred source of tritium for the preparation of labeled molecules because of its high isotopic purity. Tritium labeling allows the direct incorporation of radiolabel into drug candidates without substantial alteration of their chemical and physical properties and biology. However, many reliable hydrogenation or hydrolysis reactions cannot be applied to tritium labeling due to lack of reagents, low molar activity, restricted functional group tolerance, or safety concerns. Deuterated water (3H2O) is problematic because the label washes off quickly from the ubiquitous water and there are safety concerns because of the potential for rapid absorption of radioactive water by the experimenter. However, non-homogeneous catalysts such as palladium loaded on carbon work through a reaction mechanism that also results in a reduction of other distinctive functional groups in the drug. Since they cannot decompose dihydrogen after oxidative addition is required, homogeneous palladium catalysts can react chemoselectively with aryl (pseudo)halides, but have not been used for hydrogenolysis reactions.
【Results in brief
Tobias Ritter’s group at the Max Planck Institute for Coal Research, Germany, reports a homogeneous hydrogenolysis reaction with a well-defined molecular palladium catalyst, showing how the thianthrene leaving group can be selectively introduced into the drug by late C-H functionalization, unlike the coordination ability of the associated palladium(II) catalysts with conventional leaving groups, enabling unprecedented double hydrogen catalysis. The present work demonstrates that this unique reactivity combined with the chemoselectivity of well-defined molecular palladium catalysts enables the deuteration of small molecule drugs that contain functionality that may not be tolerated by multiphase catalysts. The deuteration reaction does not require an inert atmosphere or drying conditions, and therefore has the utility and stability to have an immediate impact on drug discovery and development. The related paper was published in Nature as “Tritiation of aryl thianthrenium salts with a molecular palladium catalyst”.
– Homogeneous hydrogenolysis with palladium catalysts
Hydrogen hydrolysis is one of the most widely studied reactions in chemistry, with many important applications ranging from biomass degradation to hydrogenolysis of other persistent halogenated pollutants. Several well-defined, homogeneous transition metal catalysts based on rhodium, iridium and ruthenium can split strong hydrogen bonds and be used in numerous productive hydrogenolysis reactions with unsaturated bonds. However, since most transition metal hydrides do not work for oxidative addition reactions of carbon-heteroatom bonds, suitable unsaturated bonds are often not present or will be destroyed by hydrogenation in pharmaceuticals, and the same hydrogenation catalysts are generally not suitable for hydrogenolysis of carbon-halogen bonds. In the case of containing dihydro and aryl (pseudo) halides, the oxidative addition reaction of dihydro is usually faster, leading to metal hydrides in a higher oxidation state, which is not suitable for the oxidative addition reaction of aryl (pseudo) halides. Therefore, for hydrogenolysis reactions of carbon-heteroatom bonds, chemists have chosen non-homogeneous catalysts, such as palladium loaded on carbon, which can effectively reduce aryl (pseudo)halides by mechanically distinct pathways. The reactivity of the active hydrogen chemisorbed on the catalyst surface leads to low chemoselectivity and undesired reduction of other functional groups commonly found in drugs (Figure 1a). After oxidative addition of the attached Pd(0), there is no suitable coordination site for dihydro coordination in the planar d8 Pd (II) tetra-coordinated complexes because the (pseudo)halide is more strongly coordinated to Pd(II) than to dihydro (Figure 1b). In cross-coupling reactions and photo-redox catalysis, aryl hafnium salts are more reactive than aryl halides and aryl trifluoromethanesulfonates.
Figure 1. palladium-catalyzed hydrogenolysis of hydrogen molecules
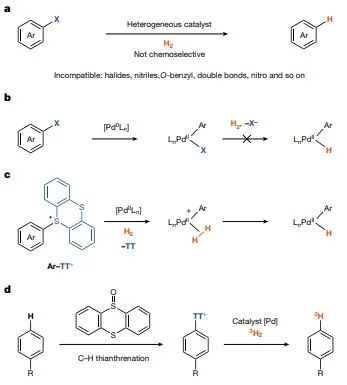
Figure 2. Homogeneous palladium-catalyzed reduction of arylthio-rhenium salts and aryl (pseudo)halides by deuteration
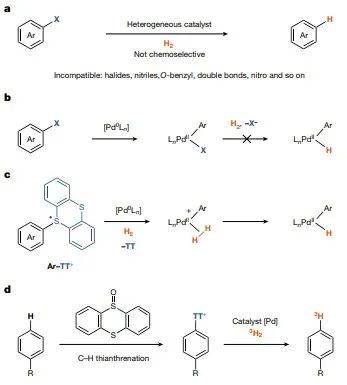
– Range of substrates for deuteration reactions
In contrast to non-homogeneous deuterium halogenation reactions, which are capable of significant isotopic displacements, a novel catalytic platform using arylthio-rhenium salts as catalysts provides a viable route for the synthesis of deuterated and trivialized drug molecules with high isotopic purity without the need for strict water or oxygen deficiency. In the present work, it was observed that for arylthio-rhenium salt starting materials, proper selection of counter ions reduces their solubility in tetrahydrofuran (THF), and as the starting materials are stored up in the solid phase, the leached material undergoes conversion as the reaction proceeds. The conversion of the hydrolysis reaction to tritium labeling reaction is carried out on the micromolar scale at subatmospheric pressure of 3H2 gas to reduce the risk of tritium gas leakage and to increase the catalyst loading to achieve faster reaction rates (Figure 4). No special attention is needed to remove air or water during the radiosynthesis process, and the radiolabeled products are easily separated from the feedstock due to the cationic nature of the thianthrene salts, which have distinct polarity differences compared to the purification after the hydrogen exchange reaction. The absence of isotopic perturbations and the straightforward purification lead to high molar activity, which is usually required for receptor binding and occupancy studies. In all cases, the high and predictable positional selectivity makes individual, well-defined, labeled molecules usually unable to react by hydrogen isotope exchange unless a guide group is used. Possible reaction pathways Finally, this work outlines a possible reaction pathway in Figure 5 that is consistent with all experimental data. The generation of catalytically active monoligand Pd(0) catalysts from the resting state Pd[(PtBu3)]2, the only observed catalytic process, is consistent with the observed reaction level of 0.5 in Pd[(PtBu3)]2. In this work, a first order kinetic isotope effect (KIE) of kH/kD = 3.1, where k is the rate constant, was determined by determining the initial rates of independent reactions with H2 and 2H2, respectively, at the same pressure, and the equilibrium isotope effect obtained from intermolecular competition experiments with H2 and 2H2 at the same partial pressure was 1.1, which is not consistent with the oxidative addition reaction of Ar-TT+ prior to dihydrogen binding , but is consistent with the reversible dihydrogen association reaction prior to the oxidative addition of Ar-TT+. Although aryl diazonium salts are usually not accessible by late functionalization, the hydrogenolysis of biphenyl tetrafluoroborate diazonium under the reaction conditions of this work, although less efficient, is consistent with the mechanistic hypothesis of this work, since no coordination anion is produced during oxidative addition. In contrast to (pseudo)halides, thiophene groups can be selectively introduced into complex small molecules. The present work shows here how the lack of strong coordination to palladium and the intrinsic solvation properties enable anthracene chemistry to address the challenges of homogeneous palladium-catalyzed hydrogenolysis for the chemoselective synthesis of tritium-labeled small molecules.
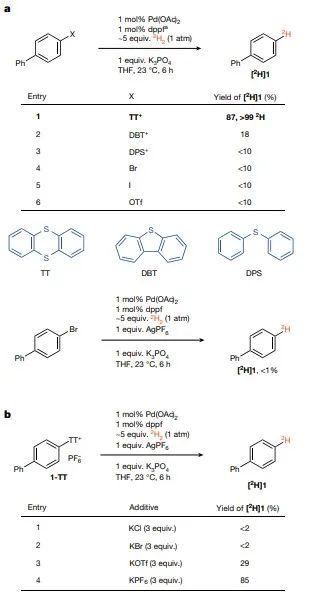
[Conclusions and Perspectives
In summary, this work reports a homogeneous hydrolysis reaction with a well-defined molecular palladium catalyst, demonstrating how the selective introduction of thianthrene leaving groups into drugs via late C-H functionalization differs from the coordination ability of traditional leaving group related palladium(II) catalysts, enabling a previously unrealized with double hydrogen catalysis. The very different reactivity combined with the chemoselectivity of well-defined molecular palladium catalysts allows deuteration of small molecules containing functionalized drugs to be tolerated by non-homogeneous catalysts. Deuterated reactions do not require inert atmospheres or drying conditions, and therefore have the utility and stability to have a direct impact on drug discovery and development.

